Exosomes are present in all cells, tissues and body fluids. They fulfill a critical role as communicators between cells, which makes them ideal candidates for an extensive range of diagnostic and therapeutic applications. However, the surface chemistry and size of exosomes often overlap with other vesicles. Traditional purification techniques can damage exosomes as they are sensitive to shear stress and do not tolerate extreme chemical conditions.
This page covers:
The purification process for exosomes is a challenging task in industrial bioprocessing as it requires a fast, accurate, and specific purification process, along with selecting the right analytical support, but CIM® monolithic columns provide a solution. The monolithic chromatographic bed is a single-unit structure, homogenously interconnected with channels. The flow through them is laminar, enabling high yields, without turbulent mixing or shearing. Large channels are easily accessible for exosomes and offer high binding capacity. The absence of dead-end pores renders mass transport relying exclusively on mass convection, eliminating the need for residence time. Monoliths come prepacked and pre-validated, saving you time and cost in your process development.
With our purification approach:
- Purify and concentrate EVs in a single chromatographic step
- Reduce other nonvesicle contaminants, especially host-cell DNA and protein impurities
- Fast process and high resolution due to convection-based mass transfer are enabled
EV Analytics
Developing your purification process requires robust analytics, ensuring that at the end of the process, your exosomes are pure, and can be further safely used for your application. With our PATfix multiple detector setup, you can monitor at-line your purification process. It allows you to track changes in impurity profiles and yields of your process, providing valuable insights for optimizing your exosome purification workflow.
Option 1: Simplified Process
A process developed for ease of use. Designed to achieve highly pure exosomes with as little handling as possible to keep them intact

Figure 1: The sample is filtered to remove major impurities, and possible dilution happens to achieve binding conditions for the chromatography step. The sample is loaded on the column and eluted with a salt gradient. Exosomes are separated by their charge properties.
Option 2: Addition of Enzymatic Treatment
A process designed to reduce the host-cell DNA impurities to a minimum. Salt-tolerant nuclease treatment of your sample reduces DNA impurities while retaining the purity and integrity of your EVs.

Figure 2: The sample is filtered to remove major impurities. Then follows the first TFF step where the salt concentration is elevated to enhance the enzymatic treatment with a salt-tolerant nuclease. Most of the host-cell DNA impurities are removed with the second TFF step. We also lower the conductivity of the sample to prepare it for loading on the CIMmultus column. The sample is loaded on the column and eluted with a salt gradient. Exosomes are separated by their charge properties.
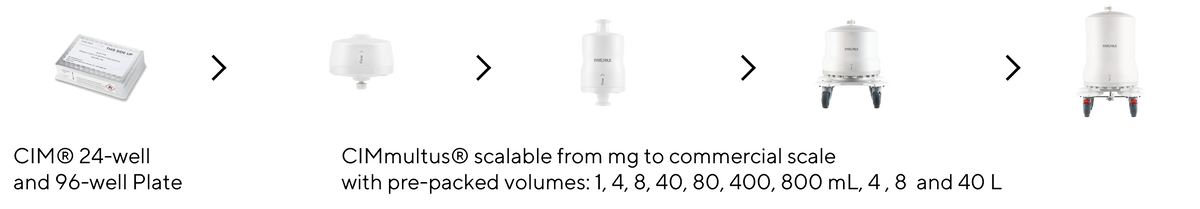
The Same Process at Any Scale
Using monoliths, you can easily transfer your process from an R&D setting to commercial manufacturing with full cGMP compliance.
Cornerstone® Biomanufacturing Development Services
Whether you are at the beginning of your process development or want to optimize your existing process, we are here to support you. With more than 20 years in downstream process development, we can identify quality attributes of your exosomes and optimize your process for the best performance. This allows for significant time, labor and opportunity cost savings, enabling you to focus on bringing your product to clinic and market.

Library
Related Events
Oops, nothing seems to match.





