Lentiviral vectors (LVV) have high potential in cell and gene therapy, representing a tool for effectively integrating information into the host cell genome. Whether applied in vivo or in vitro, achieving a high degree of product purity is essential.
With extensive experience and expertise, Sartorius BIA Separations is equipped to support customers with rapid purification process development and a diverse range of products tailored to specific applications.
The conventional purification process consists of production, clarification, chromatography, and formulation. At Sartorius BIA Separations, we focus primarly on chromatography steps.
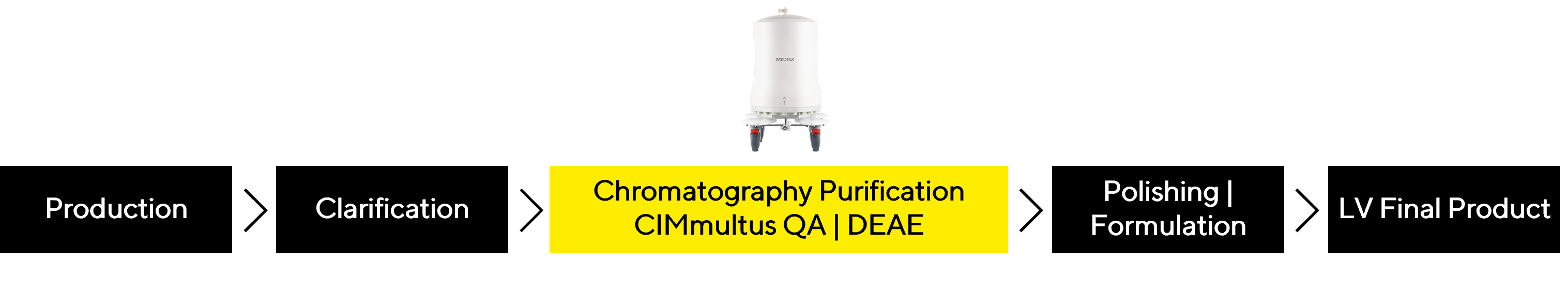
Enhancing Recovery with Insights into LVV Nature Using a Tailored Approach
The challenges one faces during the purification process are mainly connected to LVV stability; therefore, the focus is not only on final purity but also on the speed of the process and the high infectious recovery. By leveraging CIMmultus® QA technology, the innovative approach was designed to optimize the performance of LVV chromatography purification. Similarly, CIMmultus® DEAE has also shown promising results, holding high potential as an alternative.
Sartorius BIA Separations has successfully developed a chromatography purification solution on CIM® QA products that significantly enhances the process and achieves infectious titer recovery, reaching 90%. We were able to remove, on average, 3.3 log of host cell protein impurities, and significant amount of hcDNA.
Reliable Analytical Methods: Essential for a Successful Process
As part of Cornerstone® biomanufacturing development services for the purification process, we understand the critical aspects of each step and the necessity of a holistic approach backed by orthogonal analytics. Our CIM® plates enable the simultaneous screening of multiple chromatographic conditions, facilitating rapid optimization of process conditions.
The primary objective of the process is to provide a product that can integrate into the target cell’s genome with high recovery. To meet the objective, we are monitoring infectivity as well as viral genome and particle concentration. Furthermore, the removal of hcDNA and protein impurities is evaluated using hcDNA PCR, PicoGreen dsDNA Assay, HCP ELISA, and BCA Protein Assay.
To connect various analytical data into one method and have insight into sample composition is a fingerprint method using PATfix® Chromatography System, allowing identification and tracking of particles and impurities, simultaneously. Consequently, analytical methods employed are customized to meet specific requirements and support process development.
Key Highlights
- Solution: Process and Product A rapid purification step using the CIMmultus QA column was developed, focusing on LVV stability and impurity removal. This process is elevated by the Cornerstone biomanufacturing service, which employs a holistic approach and orthogonal analytics.
- Infectious Recovery Maintaining infectious recovery during the chromatography step is prioritized.
- Purity A variety of methods are implemented to monitor and verify product purity, ensuring it meets the standards required for therapeutic applications.
Library
Related Events
Oops, nothing seems to match.



