Variation in process parameters often leads to inconsistent purification results. One of the critical parameters for reproducibility is the performance of chromatographic columns, requiring consistency across batches and scales.
CIM monolith HR line is a new generation of pre-packed chromatographic monolithic products developed to meet the most demanding separation and purification needs. HR stands for High Reproducibility between batches and different scales.
CIM QA HR line includes all standard CIM column scales: CIMmultus for preparative applications, from 1 mL to 8 L, as well as CIM QA HR 96-well plates, CIM Octa QA HR, analytical CIMac QA HR columns and industrial 40L CIMmultus QA HR columns. In combination with the novel QA methods, outstanding separation of different AAV capsids for enrichment of full AAV capsid should be possible at any scale.
CIM PrimaS HR line currently features CIMmultus preparative columns. We are excited to offer additional product formats as part of our exclusive beta program. Interested in joining our beta campaign? Contact us to learn more and participate.
This page covers:
- CIMmultus QA HR Columns
- CIMmultus PrimaS HR Columns
- Novel QA High Resolution Method to Separate Different AAV Capsids
Select a Process Step
CIMmultus HR Columns
The new CIM HR line feature the same ligand as the standard CIM columns, but implements an additional specialized testing procedure with strict release criteria.
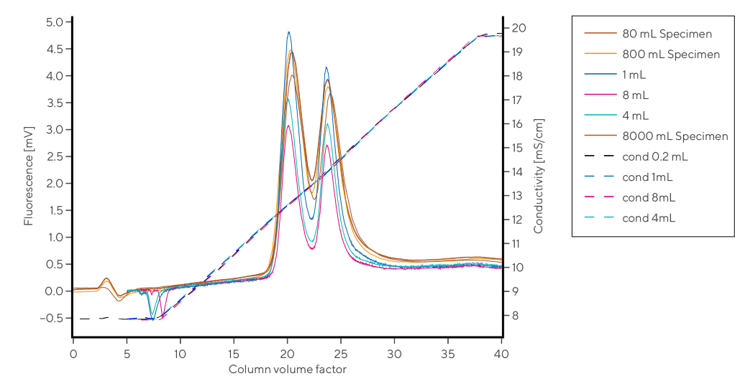
CIMmultus QA HR
CIM monolith QA HR provides reproducible purity allowing for enrichment of any full AAV capsids and its chimeras, or surface modified capsids, regardless of the batch or size of the column used. Acceptance criteria of the CIM QA HR are based on the conductivity values of the empty AAV capsids elution, instead of the ion capacity test using small ionic molecules applied for the standard CIM QA.
*The new QA method is patent pending but already available as part of Cornerstone® Biomanufacturing Services.
CIMmultus PrimaS HR
CIM monolith PrimaS HR enables reproducible column performance regardless of the batch or size of the column used. Acceptance criteria of the CIM PrimaS HR are based on the pH values of the empty AAV capsids elution in ascending pH gradient.

As the production methods are the same, the quality assurance regulatory support file for the CIM HR cGMP columns is also the same as for the standard cGMP product line. This allows users of cGMP compliant columns a smooth transition from the standard line to the new HR line.
Novel QA High Resolution Method to Separate Different AAV Capsids
The presence of partial and heavy AAV capsids in AAV gene therapy products is an increasing concern due the potential carryover of host cell DNA (hcDNA) and plasmid DNA (pDNA) fragments.
By using recently developed separation methods in combination with CIMmultus QA HR columns, it should be possible to remove one log of partial and heavy capsids and about two orders of magnitude of the empty AAV capsid without sacrificing much of the product at any scale.
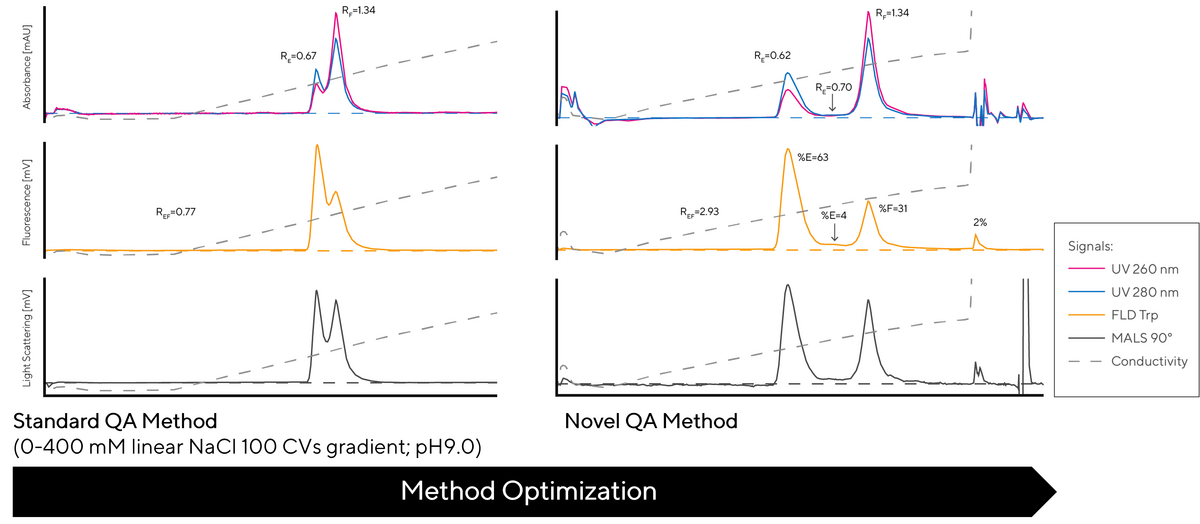
Figure 2 shows a difference between the standard and new QA methods. The two methods were carried out using the same strong anion exchange CIMac QA HR analytical column applying the same CIMmultus SO3 pre-purified sample. The novel QA method provides significantly improved resolution between full capsids and other non-active, potentially unsafe capsids.
The chromatograms show elution profiles of both methods monitored by three different detectors, UV (at 260 nm and 280 nm), Trp Fluorescence and Light Scattering.
Example of CIMmultus QA HR Chromatogram
Cornerstone® Biomanufacturing Services
This service typically consists of 2 steps:
- Feasibility Study First, the Feasibility study is performed to demonstrate that the method provides the expected high resolution separation using client’s AAV sample. Outcome of this study is a proof of principle and consists only of chromatograms and a basic interpretation of the characteristics of the sample based on the chromatograms.
- Full Study After the feasibility study, one can proceed with the paid full study using the novel empty-full AAV method, and the client receives a worldwide nonexclusive, free of charge license for using novel QA empty-full method.
Related Events
Oops, nothing seems to match.




