Modes of flow and mass transport
Flow patterns are important in chromatography media because they determine binding and elution efficiency, peak dispersion, eluted product concentration, and shear stress. One of the most important points to remember when trying to understand how chromatography media work is, Water always takes the path of least resistance!
Mass transport through chromatography media can be convective, diffusive or both, depending on structure:
- Convection is defined as movement induced by an external force. In chromatography, that external force may be gravity or a pump.
- Diffusion is defined as random thermal movement from an area of high concentration to an area of low concentration.
Both of these definitions represent unattainable ideals with adsorptive media because attractive and/or repulsive forces between stationary phase ligands and solutes are highly influential. However, an understanding of mass transport is still required to appreciate the performance differences among media formats.
Continuity of Flow
Movement of solutes through monoliths is continuous which means there is only one mode of flow throughout the entire bed - mass transport in monoliths is exclusively convective. Flow through columns of porous particles is discontinuous. Mass transport into and out of pores is diffusive while flow between particles is convective.
Benefits of Convection
Convective mass transport is the most intuitive mode and has major benefits. As all solutes flow with the current regardless of their size a 1 nm peptide and a 100 nm virus particle are transported at the same rate (Image 1). With exclusively convective mass transport, monoliths are able to maintain sharp peaks regardless of solute size. Resolution amongst peaks is also flow rate independent which is possible partly by elution occurring strictly as a function of mobile phase composition, while in porous particles elution occurs in two steps: first the chemical dissociation of the solute from the ligand and then solute diffusion out of the pores. With increasing flow rate, for porous particles, peaks will broaden as diffusive mass transport becomes less efficient. The broadening factor increases with solute size and flow rate.
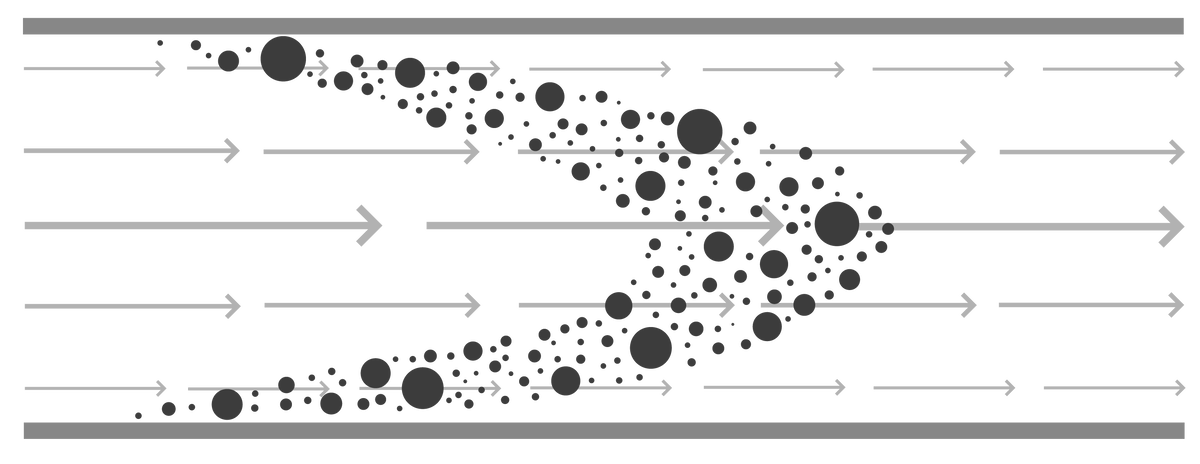
Convective mass transport also provides corollary benefits that are less obvious. Where affinity chromatography with porous particles typically requires several minutes of sample residence time in the column to achieve the best capacity, monoliths require a few seconds. Where solid phase enzyme bioreactors on porous particles suffer low efficiency due to the long time intervals for diffusion to bring the substrate in contact with the enzyme, kinetic efficiency of monolithic bioreactors closely approaches the efficiency of the enzymes in free solution.
Limitations of Diffusion
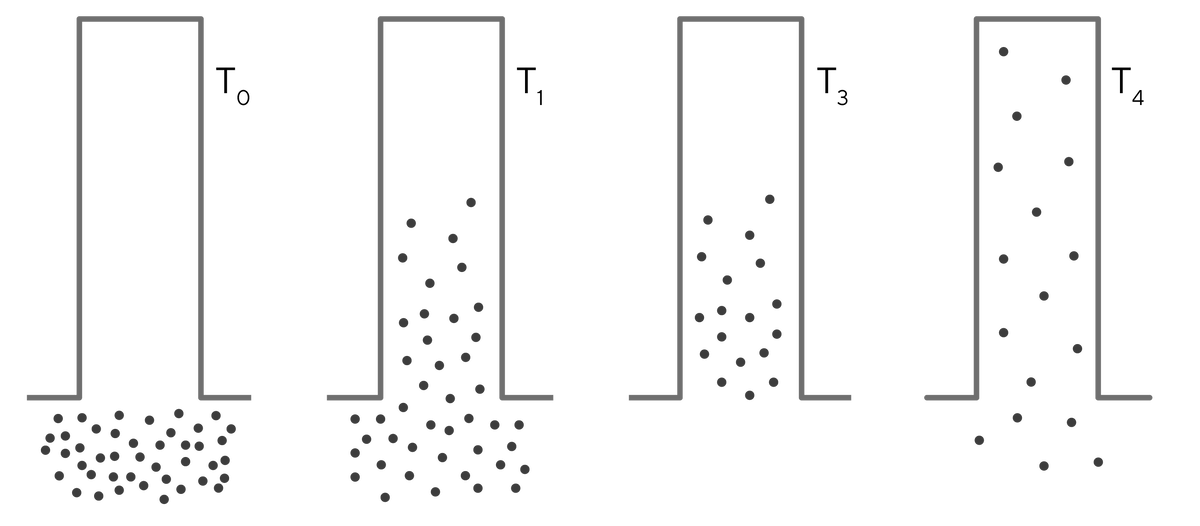
Diffusion in unrestricted space is also intuitive: solutes disperse until equilibrium is reached. Larger solutes have slower diffusion constants so they diffuse and reach equilibrium more slowly. Movement in restricted spaces gives the false impression of diffusion being directional but it is not. It is driven strictly by concentration gradients (T0 and T1 on Image 2 ). Solutes do not diffuse entirely into pores. When equilibrium is reached, the concentration inside and outside the pores is the same (T1). If external solutes are removed (T3), solutes inside a pore can diffuse more deeply into a pore as well as out (T4). This contributes to product losses and carry-over in porous particle columns, especially at high flow rates.
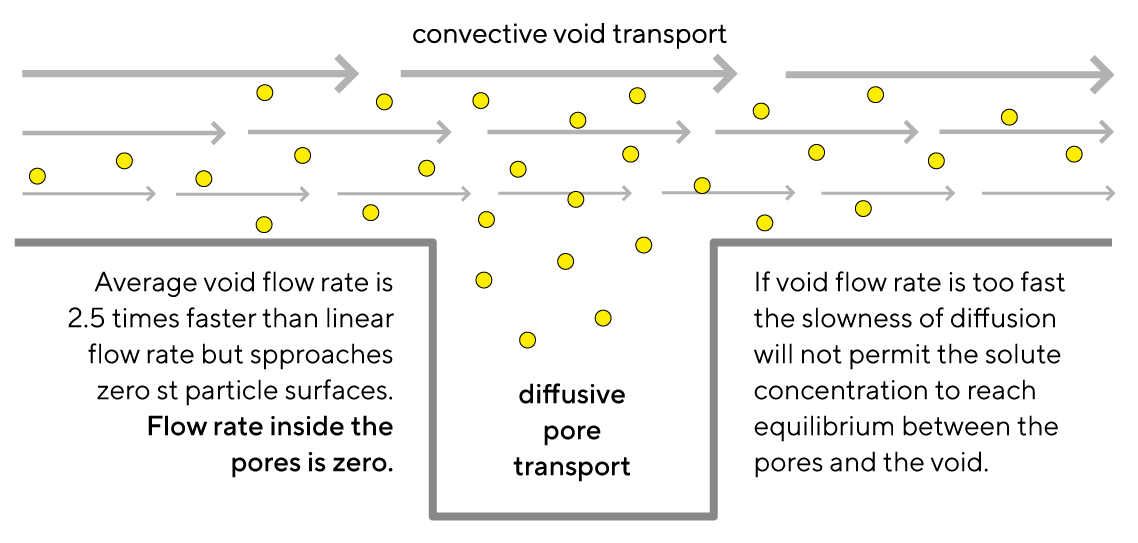
Net diffusive efficiency is highly influenced by void flow rate:
 Continue to "Modes of dispersion".
Continue to "Modes of dispersion".
