Architecture of chromatography media and devices
Get in the habit of thinking about how the architecture of chromatography devices affects their performance. It will enhance your ability to choose the chromatography format that will best serve your fractionation needs. It will provide you with a rational basis to evaluate new media and formats as they enter the market. Best of all, if you want to develop new chromatography methods, it will enable you to select the format that will give your application the best chance of success.
Monoliths
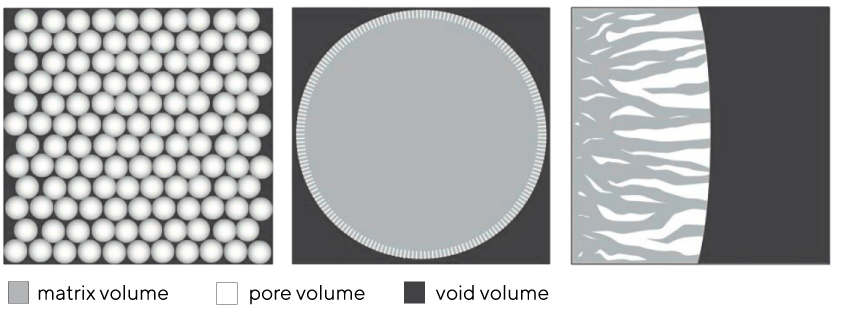
Monoliths can be defined as single-unit structures with highly interconnected convective channels distributed homogeneously throughout the entire bed. They have two structural features: the matrix volume and the channel volume. CIM monolith matrix is composed of polymethacrylate. The standardized channel size of polymethacrylate monoliths lies in the range of 2 μm, offering also 1.3 μm and 6 μm as custom made, to accommodate varying size of the solutes to be fractionated and the contents of the feedstream. Big channels allow for unhindered passage of all commonly used large biomolecules. This permits high flows with low pressure drops.
On average, every channel in monolith is connected to sixteen or more others. A corollary of high connectivity is that there are no dead ends. High connectivity contributes to even flow distribution and reduced operating pressure. It also makes the entire channel surface area available for binding, leading to higher capacity. Moreover, absence of dead-ends avoids entrapment of solutes. Dead-ended media can cause product losses, fouling, and carry-over between runs.
Columns packed with porous particles
Columns packed with porous particles are based on the porous bead filing. Internal volume of pores is referred to as the pore volume and there is where the majority of the column's solute binding sites are located. Solute-accessible pores are restricted to the outer strata of the particles with average pore sizes for most media used to fractionate biomolecules range from about 50 to 125 nm. Connectivity among pores is generally nil, with an average of about 1.5. This translates to half the pores being connected to no others, and most of the rest having a single connection to one adjacent pore. All the pores are dead-ended which together with poor pore cross-connectivity shows in significant performance limitations (discussed in-depth in next chapters) (Image 2).
The spaces between particles are collectively referred to as the void volume; individually as void compartments. The total void volume in all particle columns is ~40% of the bed. This also means the average size of individual void compartments is ~40% of the average particle size. Such irregular cavities in flow streams of particle-based columns create large vortexes causing severe shear and consequential loss of recovery.