Capacity as a function of structure and transport
Binding efficiency is determined primarily by the dominant mode of mass transport in a chromatography device. Convective mass transport gives sharp near-vertical solute breakthrough curves at all flow rates. Diffusive mass transport on the other hand, produces extended sigmoidal curves that degenerate with increasing flow rate. Total binding surface area for small solutes is greater with porous particle columns than with monoliths, but most of it resides inside the pores and diffusion is the only way in or out.
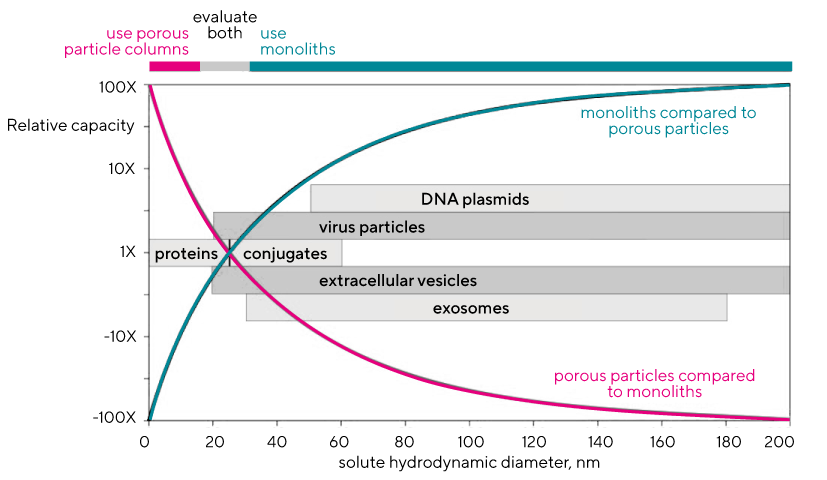
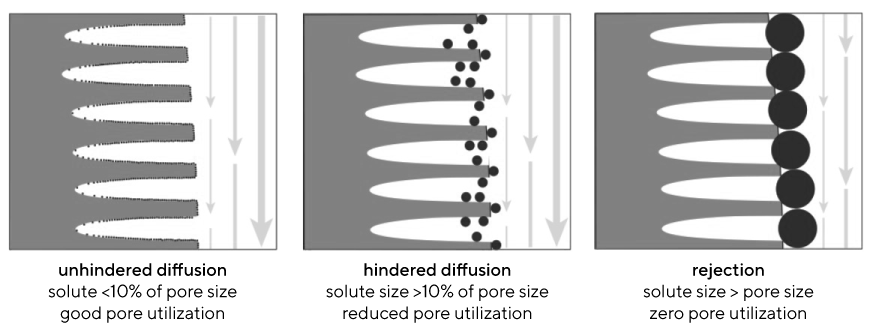
Monoliths support higher capacity for large solutes (i.e., viruses and DNA) at all flow rates, whereas porous particles support higher capacity for smaller solutes (i.e., proteins) at low flow rates. Breakthrough curves are also indicators of media utilization. Ineffective column utilization is attributable mainly to the low efficiency of diffusive mass transport. It becomes more severe at higher flow rates and with larger solutes (Image 1). Monoliths lack these limitations and support higher capacities for large solutes at all flow rates, whereas porous particles support higher capacity for smaller solutes (i.e., proteins) at low flow rates.
Hindered diffusion is another major piece of the puzzle in porous particle media inefficiency. It refers to a situation in which obstacles interfere with the random movement of solutes, in this case the pore walls. It compounds the fundamental inefficiency of diffusive mass transport. Monoliths support higher capacity for large solutes because access to the binding surface is not hindered.
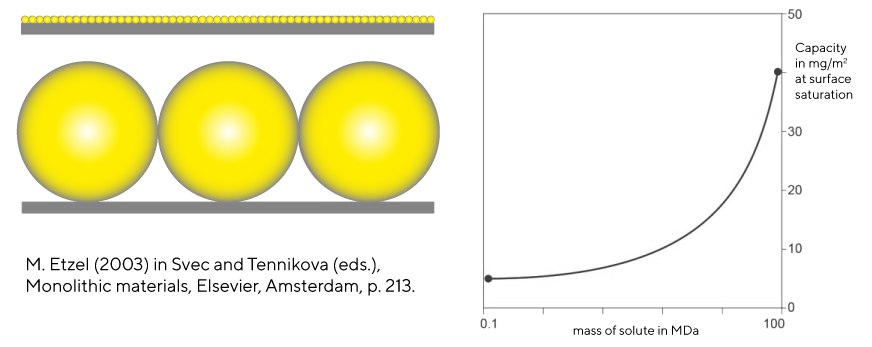
Monoliths also support higher capacity in mass per unit of surface area because solute mass increases more as a function of its three-dimensional size than as a function of the surface area it occupies (Image 2). Porous particle columns cannot benefit from this relationship because the slower diffusion constants of larger solutes progressively restrict them from achieving surface saturation. Monoliths therefrom, offer optimal solution for large biomolecules as they (relatively) offer largest capacities for the largest of the commercially interesting biomolecules (Image 3).