Overview of Cornerstone® Biomanufacturing Development Services
Are you seeking speed and scalability for your virus particle and large molecule process, from preclinical and PI to PIII and beyond?
Do you lack expertise and capabilities when it comes to AAV, pDNA or mRNA purification and analysis?
Are you looking to improve or fix an existing large biomolecule process?
No matter if you are a large company or a start-up. We have got your back!
Downstream part of the process is a complex matrix full of interdependent parameters where optimization is not straightforward, so experience provides the quickest way to identify the key quality attributes for your unique product and optimizing them for best performance. This allows for significant time, labour and opportunity cost savings, permitting your team to focus on bringing your product to clinic and market.
Our Cornerstone® Biomanufacturing Development Services are result of 25 years of hands-on experience with the most challenging biopharmaceutical products and offer a comprehensive approach of integrated process development solutions and novel technology designed to improve the robustness and yield of AAV, pDNA, mRNA, exosomes and other Advanced therapy medicinal products (ATMPs) production, while improving the safety of therapeutic products.
 Manufacture of plasmids (E.coli) Plasmid range: 1 – 30kbp Bioreactor scale: 10L & 50L ■ DSP Purification of plasmid RNase-free process |  Linearization optimization, scale-up, IPC analytics ■ IVT IVT optimization IPC analytics, scale-up ■ DSP Two-step purification mRNA Scale-up, IPC analytics Purity within regulatory spec |
 AAV Transfection of plasmids/Adeno upstream (HEK) Bioreactor scale: up to 5 - 10L ■ DSP Purification of viruses (AAV, Adeno, vaccina, influenza, etc.) Scale-up Dedicated analytical methods |
 Purification EV MALS detection Dedicated exosome analytics |  Phage expression Bioreactor scale: up to 50L ■ DSP Purification of phages >8log endotoxin removal |
 | |
 | BSL 1 & 2 labs (non-GMP) Single step or full process Reports: SOP/results Product quality & quantity Monitoring purification process Process fingerprints & impurity profiles Characterization of raw materials Method validation Technology comparisons Custom-made monolith columns |
 | PATfix® LC analytics UV/FLD/MALS CGE/AGE/SDS-PAGE NTA/UAC/UV/MALS Dot-blot/μBCA ELISA/qPCR/ddPCR/TCID |
 | Relative titre and conc Purity, yield, MW, radius Contaminant profile (i.e. HCP/gDNA, etc.) Full/empty particles (AAV/Ad/VLP) Quantitative OC/SC (plasmid) Quantitative: mRNA, IVT components Integrity of mRNA |
 | |
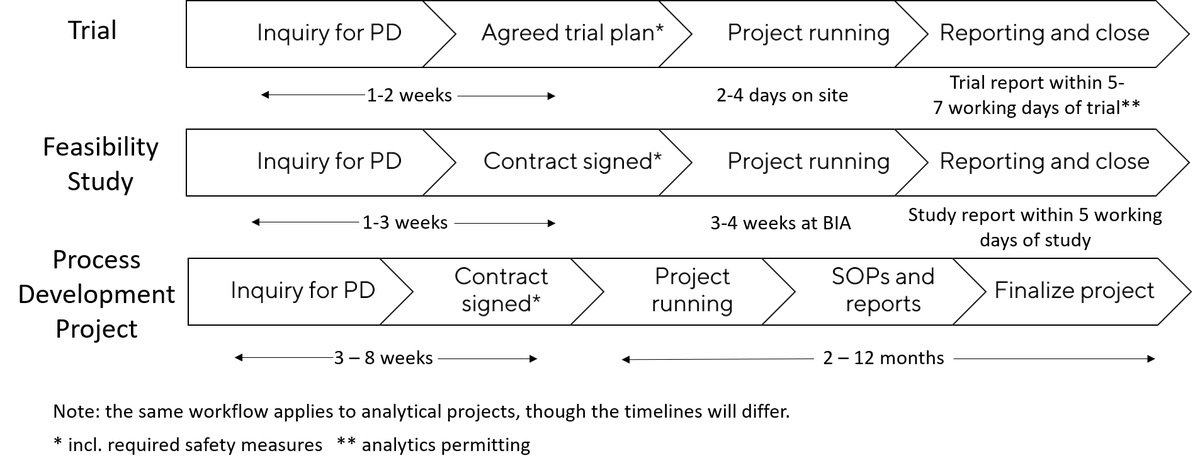
 | Single step or full process Proof of concept (no optimization) Short report Timeline: 1 - 2 months |
 | USP, DSP or both Single step or full process Full report: results/SOP/tech transfer Tech transfer support (CDMO) Timeline: 4 – 12 months |
Reach out to us and have your Process Developed in 4 simple steps.
In our expanded, fully-equipped BSL 1 and 2 facilities, we can support you with process development, analytical method development, or preparation of custom purification media with your ligand of choice.
 | Explain your needs, fill out the questionnaire. Let's identify together your goals and set up a work plan. |
 | Based on your needs and scope, we prepare an informative offer and all the documentation needed. |
 | After confirming the plan and signing the contract, just send us your sample and material and we will get busy. |
 | Time is of essence so we will do our best to develop the process for you, including SOPs, training, transfer, scale-up , on site support in timely manner. |
Better purification process. Faster.
Cornerstone® Biomanufacturing Development Services solve the challenges and accelerate process development from pre-clinical to market supply. Our objective is to give the customers resources, knowledge, technical solutions and their product - in time and in the right quality.