- Are you seeking speed and scalability for your virus particle and large molecule process, from preclinical and PI to PIII and beyond?
- Do you lack expertise and capabilities when it comes to AAV, pDNA or mRNA purification and analysis?
- Are you looking to improve or fix an existing large biomolecule process?
Cornerstone® Biomanufacturing Development Services offer integrated process development solutions and novel analytics technology for large biomolecules such as viral vectors, viruses, nucleic acids, exosomes and bacteriophages. Our team is skilled in complete processes suitable for cell and gene therapy, vaccines and other therapeutic areas and will help you with different development phases up to market supply, including tech transfer, SOPs and validated analytical methods.
No matter if you are a large company or a start-up, we’ve got your back!
In our expanded, fully-equipped BSL 1 and 2 facilities, we can support you with process development, analytical method development, or preparation of custom purification media with your ligand of choice.
Supported Modalities
Nucleic Acids — plasmid DNA (pDNA), messenger RNA (mRNA)
Nanoparticles — Lipid Nanoparticles (LNP)
Viral Vectors — Adeno-associated virus (AAV)
Our Track Record
30
DNA, RNA and virus processes have been tech transferred to CDMOs
40
Large molecule projects have been successfully tech transferred to CDMOs
60
Simultaneous projects can be run in parallel at various stages of development and complexity
The Timelines and Workflow
- Feasibility Study:
Start with a feasibility study to establish proof of concept without the optimization of work or robustness checks. The study usually takes 1 month or less and provides a brief report with purification data and results. Usually, one scientist is involved.
- Process Development Project:
Continue with a comprehensive process development project that typically spans 2 to 12 months and provides detailed reports, SOPs, training, and tech transfer as well as optimization and scalability|reproducibility validation. The project manager and team implement new techniques and methods to ensure success.
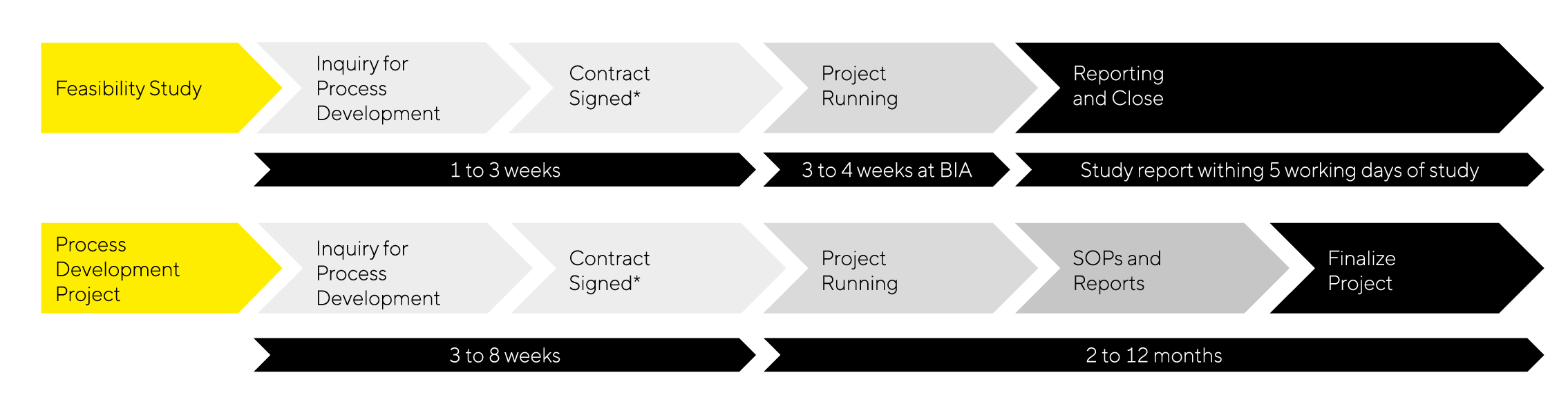
Note: The same workflow applies to analytical projects, though the timelines will differ.
*incl. required safety measures
| Package Option | Feasibility Study | Process Development |
| Timeline | 1–2 months | 4–12 months |
| Scope | Proof of concept: no optimization or robustness checks |
Process optimization, scalability, and reproducibility validation |
| Single Step | ✓ | ✓ |
| Full Process | ✓ | ✓ |
| USP | ✓ | ✓ |
| DSP | ✓ | ✓ |
| Deliverables | Short Report | Full Report: results, SOPs, training, tech transfer |
Have Your Process Developed in 4 Simple Steps:
- Contact us Explain your needs, fill out the questionnaire. Let’s identify together your goals and set up a work plan.
- Technical Offer Based on your needs and scope, we prepare an informative offer and all the documentation needed.
- Execution After confirming the plan and signing the contract, just send us your sample and material and we will get busy.
- Tech Transfer Time is of essence so we will do our best to develop the process for you, including SOPs, training, transfer, scale-up , on site support in timely manner.
With over 25 years of experience we are able to provide you with the quickest way to identify the key quality attributes for your unique product and optimize them for best performance. This allows for significant time, labor and opportunity cost savings, permitting your team to focus on bringing your product to clinic and market.
Do You Have a Question?
FAQ About Cornerstone Services
The primary focus is on chromatography, specifically using monolithic columns. Within Cornerstone Biomanufacturing Development Services, technologies such as tangential flow filtration (TFF), filtration, and clarification are also addressed. In Cornerstone Biomanufacturing Development Services, the focus is on the in-house developed liquid chromatography (LC) system, complemented by a variety of different and orthogonal analytical and biochemical methods.
Yes, purification processes are highly scalable for commercial production due to the use of advanced monolithic chromatography columns. These columns are available in a variety of sizes, ranging from 1 mL to 40 L, and feature channel sizes of 1.3μm, 2μm, or 6μm. Offered in both cGMP and non-GMP compliant formats, they support a broad spectrum of chemistries, including ion exchange (IEX), hydrophobic interaction (HIC), and affinity chromatography. For screening applications, a small CIMmic disk with a 0.1 mL bed volume and 2μm channel size is available. The scalability from 1 mL to 40 L makes these processes ideal for research, screening, process development, pilot projects, and full-scale manufacturing.
Cornerstone Biomanufacturing Development Services offers Process Development, Analytical Development, and Custom Monolith Development and Manufacture.
Purifying large biomolecules, especially those used in cell and gene therapy (CGT) and vaccines, presents unique challenges due to their delicate nature and distinct behaviour compared to traditional monoclonal antibodies (mAbs). These innovative biomolecule modalities, such as viral vectors, nucleic acids, and viruses, often require specialized purification techniques, as conventional methods may not be sufficient. Additionally, the lack of established purification platforms and comprehensive process monitoring, and analytics further complicate the purification process. Cornerstone Biomanufacturing Development Services excel in overcoming these challenges by providing state-of-the-art solutions for the production, purification, and analysis of large biomolecules. The services facilitate the swift and efficient integration of these solutions into customer processes, ensuring effective, robust, and scalable purification outcomes.
Support and expertise during the development and implementation of purification processes are provided through a combination of resources, knowledge, and technical solutions, ensuring timely delivery of high-quality final products. Leveraging extensive experience in cell and gene therapy, vaccines, and other therapeutic areas, the focus is on large biomolecules like viral vectors, viruses, nucleic acids, exosomes, and bacteriophages. With unmatched expertise in CIM monoliths, the services accelerate customers’ path to clinical trials, quickly deliver scalable and robust processes, and improve yield and purity. Our Cornerstone Services also help reduce process costs (COGs) and provide rapid, accurate analytics for product and process monitoring and control. This approach optimizes outcomes for monolith-based processes and can lead to the development of new monoliths to better serve unmet needs and emerging molecules.
To initiate discussions with a customer, a Non-Disclosure Agreement (NDA) is typically required. This NDA is often on the side of the customer to protect their material, knowledge, and intellectual property (IP). If an NDA is not already in place, Sartorius has templates available to expedite the process. Additionally, before any material is shipped, a Material Transfer Agreement (MTA) must be signed. For more comprehensive projects, a Master Service Agreement (MSA) or a work order may also be necessary, especially if the project is technically challenging and could generate new IP.
Over 50 full-time employees at Sartorius BIA Separations are dedicated to Cornerstone Biomanufacturing Development Services. The team is organized into three main organizational units. Team members not only work on customer projects but also engage in internal research for new product, process, and method development. Additionally, they are involved in technology exploration, training, education, and patent work.
Cornerstone Biomanufacturing Development Services focus on surpassing the minimum standards set by regulations. They prioritize meeting and exceeding the specific purity criteria established by customers, which has proven advantageous in the past. This approach involves implementing advanced purification techniques, rigorous quality control measures, and continuous process optimization to achieve purity levels higher than those mandated by regulatory bodies.
We have successfully collaborated with several companies. You can read what our partners say about us here. We have collected highlights from their feedback:
“We are especially grateful that BIA Separations shared, and operated, with the same sense of urgency we did to help bring gene therapy to the SMA community. BIA’s experience with AAV purification and its chromatographic technology were important contributions and we look forward to our continued work together.” – Andy Stober, Senior Vice President of Technical Operations, AveXis (Now Novartis)
“The human capital at BIA Separations is amazing. People are dedicated; it’s not only the column, it’s basically the people behind it that can help you improve your production and analytics at every step. From a CMC standpoint, this is super important. I don’t think there is any other company that can do that. The know-how at BIA is really amazing, and the ability to look at pure products at the end with analytical tools is what makes them different from everyone else.” – Dan Peer, Vice President for Research and Development, NeoVac
“We are very pleased to be collaborating with BIA Separations / Sartorius in developing and tech-transferring optimized mRNA vaccine manufacturing processes. When time is of the essence, it is crucial to be able to rely on key partners whilst maintaining the highest level of quality in every aspect of our work. We are also very proud that our team was able to produce two kinds of genetic vaccines (DNA and mRNA) and we look forward to expanding our partnership with the BIA Separations’ team on future projects.” – Hong Thai Pham, CEO, BioNet
Consult Our Experts
Our experts would be happy to discuss your project. Fill the contact form below or send us an email to cornerstone@biaseparations.com

