Which Modality Can We Assist You With?
Where Innovation Meets Expertise | The Tour of Our Labs
The state-of-the-art facilities are fully equipped to support both upstream (USP) and downstream processing (DSP), ensuring that we deliver the highest quality results. At the heart of our operations is the monolithic technology, which enables the superb separation of biomolecules.
A team of knowledgeable and inventive experts is dedicated to the development of processes for viruses, exosomes, and virus-like particles.
We are committed to developing effective, fast, and robust processes for target molecules, ultimately serving patients in need of gene therapy, oncolytic treatments, or safe vaccines.
Have Your Process Developed in 4 Simple Steps
- Contact us Explain your needs, fill out the questionnaire. Let’s identify together your goals and set up a work plan.
- Technical Offer Based on your needs and scope, we prepare an informative offer and all the documentation needed.
- Execution After confirming the plan and signing the contract, just send us your sample and material and we will get busy.
- Tech Transfer Time is of essence so we will do our best to develop the process for you, including SOPs, training, transfer, scale-up , on site support in timely manner.
With over 25 years of experience we are able to provide you with the quickest way to identify the key quality attributes for your unique product and optimize them for best performance. This allows for significant time, labor and opportunity cost savings, permitting your team to focus on bringing your product to clinic and market.
Timelines and Workflow
- Feasibility Study:
Start with a feasibility study to establish proof of concept without the optimization of work or robustness checks. The study usually takes 1 month or less and provides a brief report with purification data and results. Usually, one scientist is involved.
- Process Development Project:
Continue with a comprehensive process development project that typically spans 2 to 12 months and provides detailed reports, SOPs, training, and tech transfer as well as optimization and scalability|reproducibility validation. The project manager and team implement new techniques and methods to ensure success.
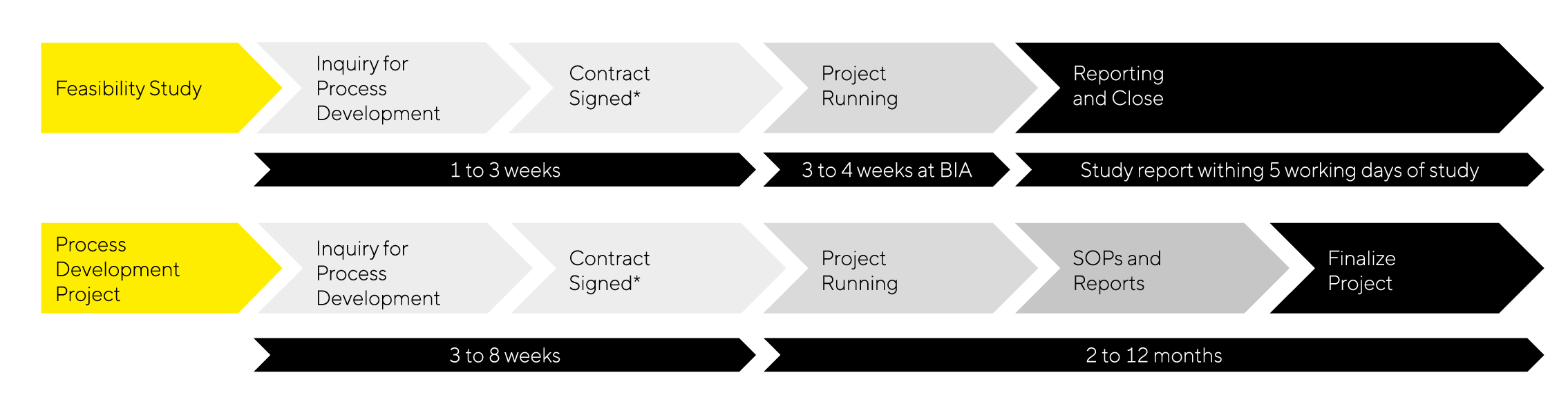
*incl. required safety measures
Hear from Our Customers
Do You Have a Question?
FAQ About Services for
Sartorius BIA Separations provides cornerstone services to a variety of industries, including biotechnology, pharmaceuticals, diagnostics, and life sciences, with specialized expertise in:
- Viral vectors: We offer tailored solutions for the purification and analysis of a wide range of viral vectors, including AAV, adenovirus, lentivirus, influenza virus, bacteriophages, NDV, vaccinia virus, poxvirus, KHV, chikungunya virus, Lassa virus, VSV, MVM and many other. These services support applications in gene therapy, vaccine production, and research, ensuring high purity and yield while meeting customers requirements.
- Virus-Like Particles (VLPs): Our services enhance the development and manufacturing of VLPs, crucial for vaccine development, by optimizing purification processes to achieve high-quality and scalable production.
- Extracellular Vesicles: We assist companies in the isolation of exosomes for therapeutic and diagnostic applications, providing expertise in purification techniques that maintain the integrity and functionality of these extracellular vesicles.
- Lipid Nanoparticles (LNPs): Our offerings include solutions for LNPs, essential for the delivery of mRNA and other nucleic acids, ensuring stability and efficacy in drug delivery systems.
These services are specifically designed to address the unique challenges and regulatory standards of each sector, ensuring optimal performance and compliance.
Sartorius BIA Separations offers cornerstone services such as process development, purification solutions, analytical testing, and regulatory support to enhance efficiency and quality across various stages of product development. We provide expert guidance and can transfer our methods to CDMOs, along with SOPs, training for personnel, transfer, scale-up and on-site support to ensure smooth implementation of our technologies.
To get started with BIA Separations’ services, you can contact us through the contact form on our website or Cornerstone Service email (cornerstone@biaseparations.com). We’ll discuss your specific needs and goals to tailor our services to your requirements and you will receive a detailed proposal outlining the services and solutions we can provide. Once you agree to the terms, we’ll finalize the contract, and our team will begin working with you to implement the services and support your project.
To initiate discussions with a customer, a Non-Disclosure Agreement (NDA) is typically required. This NDA is often on the side of the customer to protect their material, knowledge, and intellectual property (IP). At the customer’s request, we implement Material Transfer Agreements (MTA) and/or Master Service Agreements (MSA) to clearly outline the terms for data and sample handling. Furthermore, our practices adhere to industry standards and regulations, including ISO 9001, to guarantee data protection and quality management.
- Email: Send your feedback directly to our customer service email address or person you are in contact with.
- In-Person or online Meetings: Discuss your feedback during scheduled meetings with our team.
We value your input and use it to continuously improve our services.
Consult Our Experts
Our experts would be happy to discuss your project. Fill the contact form below or send us an email to cornerstone@biaseparations.com