Cornerstone Biomanufacturing Development Services specialize in accelerating process development for plasmid DNA (pDNA), messenger RNA (mRNA), and other nucleic acid-based therapeutic modalities (saRNA, tRNA, ssDNA), addressing production challenges with advanced chromatographic analytics. Process development services deliver scalable, robust processes that comply with regulatory purity standards.
Which Modality Can We Assist You With?
For plasmid DNA, development includes fermentation, lysis optimization, clarification, TFF, chromatography, and sterile filtration. Scale-up to 10 L fermentor scale can be performed. QbD approach including DOE is applied in development.
For mRNA production, IVT reaction can be performed from 100 µL to 100 mL scale, focusing on in vitro transcription (IVT) optimization to reduce costs and minimize dsRNA formation using DOE approach coupled with PATfix monitoring. Process development maximizes IVT yield and optimizes capture steps for recovery and yield, focusing on dsRNA removal. TFF is applied for drug substance formulation. Process Development Support extends to other RNA modalities, including saRNA and circRNA.
Detailed reports and SOPs are produced as outputs of development, which ensure seamless tech transfer to customer site or selected CDMO, empowering customers with the tools and knowledge for successful nucleic acid production.
Timelines and Workflow
- Feasibility Study:
Start with a feasibility study to establish proof of concept without the optimization of work or robustness checks. The study usually takes 1 month or less and provides a brief report with purification data and results. Usually, one scientist is involved.
- Process Development Project:
Continue with a comprehensive process development project that typically spans 2 to 12 months and provides detailed reports, SOPs, training, and tech transfer as well as optimization and scalability|reproducibility validation. The project manager and team implement new techniques and methods to ensure success.
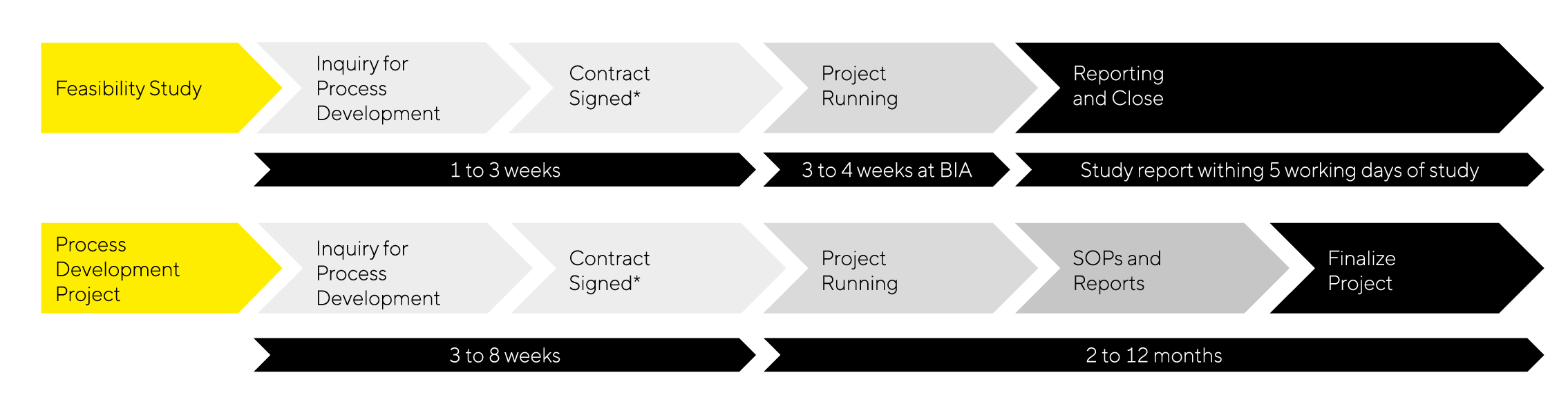
*incl. required safety measures
Have Your Process Developed in 4 Simple Steps
- Contact us Explain your needs, fill out the questionnaire. Let’s identify together your goals and set up a work plan.
- Technical Offer Based on your needs and scope, we prepare an informative offer and all the documentation needed.
- Execution After confirming the plan and signing the contract, just send us your sample and material and we will get busy.
- Tech Transfer Time is of essence so we will do our best to develop the process for you, including SOPs, training, transfer, scale-up , on site support in timely manner.
With over 25 years of experience we are able to provide you with the quickest way to identify the key quality attributes for your unique product and optimize them for best performance. This allows for significant time, labor and opportunity cost savings, permitting your team to focus on bringing your product to clinic and market.
Hear from Our Customers
Ready to Discuss The Project?
Consult Our Experts
Our experts would be happy to discuss your project. Fill the contact form below or send us an email to cornerstone@biaseparations.com