Plasmid DNA (pDNA) plays a vital role in the production of mRNA, AAV, and other therapeutic vectors, including vaccines and gene therapies.. Ensuring pDNA purity and selecting the correct isoform are crucial for these applications. Effective workflows and precise analytical methods are essential for producing high-quality pDNA, free from contaminants and meeting therapeutic standards. Advances in these methods are key to successful pDNA production and quality control. Sartorius BIA Separations experts provide comprehensive support in these areas, ensuring optimal results.
Common Challenges in pDNA Analytics
- Quantification Limitation: Conventional methods like agarose or capillary gel electrophoresis (CGE) separate pDNA from contaminants but do not quantify pDNA isoforms, which is essential for meeting regulatory quality specifications.
- False Quantification: Spectroscopic methods for quantifying nucleic acids can produce inaccurate results due to impurities that absorb UV light. Additionally, the pH and conductivity of the matrix can lead to incorrect pDNA concentration measurements.
- Unpredictable Linearization: Plasmid linearization using restriction enzymes can be uncertain, with variability in the required enzyme amount and reaction time.h variability in the required enzyme amount and reaction time.
With Cornerstone® services, these common challenges in pDNA manufacturing are addressed while providing solutions tailored to specific needs.
Overcoming pDNA Analytical Challenges
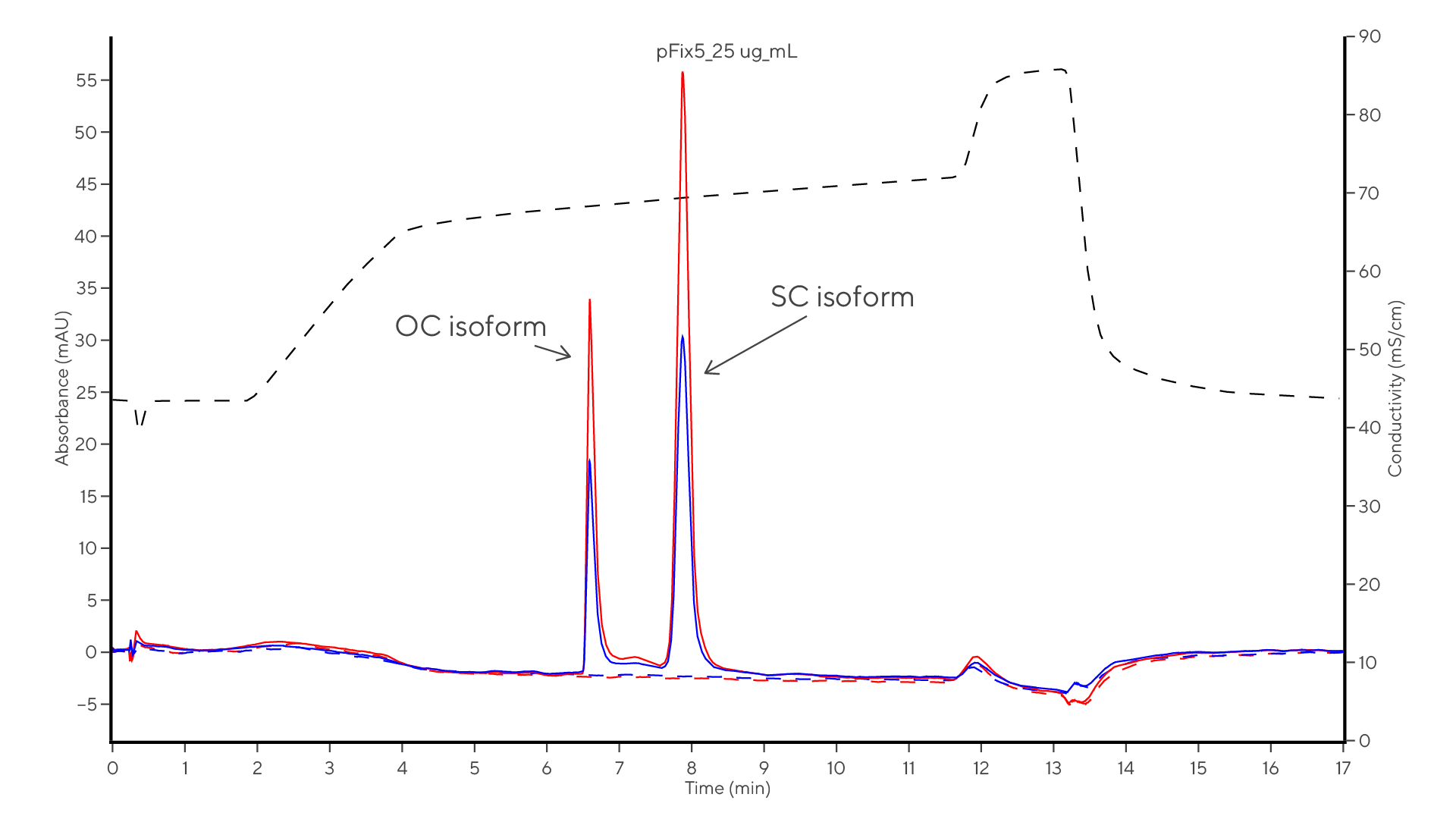
Integration of PATfix® analytics and complementary methods including CGE, delivers a comprehensive evaluation of pDNA samples. It allows for the quantification of different isoforms and identifying impurities.
The PATfix pDNA Platform, combined with the CIMac pDNA column, efficiently separates pDNA from contaminants and monitors pDNA isoforms during downstream processing, providing both qualitative and quantitative analyses. Additionally, this method optimizes plasmid linearization by tracking reaction kinetics, reducing time and costs, and detecting RNA impurities with high sensitivity.
- Precise Isoform Separation
Monolithic chromatography enables the separation and quantification of the target SC pDNA isoform from other impurities and isoforms. The CIMac pDNA columns, a weak anion exchanger, facilitate the separation of pDNA with a capacity of over 2000 μg pDNA/g biomass.
- Accurate pDNA Quantification
Monolithic chromatography separates pDNA from impurities, eliminating the chance of false quantification. This enables qualitative and quantitative determination of pDNA isoforms and impurities in just 17 minutes.
- Real-Time Linearization Monitoring
Plasmid linearization kinetics are monitored in real-time using PATfix pDNA analytics, optimizing the process to reduce time and cost.educe time and cost.
Why Choose Cornerstone pDNA Process Analytics Services?
By partnering with Cornerstone pDNA Process Analytics Services, the following benefits are offered:
- Optimized plasmid linearization by tracking reaction kinetics, reducing time and costs.
- Enhanced sensitivity in detecting RNA impurities.
- Effective separation of pDNA from contaminants with precise monitoring of pDNA isoforms during downstream processing.
- High-capacity CIMac pDNA columns handling over 2000 μg pDNA/g biomass.
- Rapid qualitative and quantitative analyses completed in just 17 minutes.
Additional Resources

Read the eBook PATfix pDNA Analytical Platform for Rapid Monitoring and Optimization of pDNA Production Process for more detailed insights
Hear From Our Customers
Ready to Discuss Your Project?
FAQ About Services for pDNA Process Analytics
The pDNA platform is a versatile solution capable of analyzing various pDNA samples, from alkaline lysates to purified pDNA. No pre-treatment is needed, only dilution with a loading buffer is required.
For more information about the role of analytics in the plasmid production process, follow the link below
Yes, the specially designed monolith column with 6 µm channels enables the analysis of large plasmids with excellent isoform recovery. The large channels prevent the physical entrapment of the large OC isoform while maintaining high resolution between pDNA isoforms.
As an orthogonal method to chromatographic evaluation, we offer pDNA testing by CGE, including method development tailored to the specific needs of your project.
Consult Our Experts
Our experts would be happy to discuss your project. Fill the contact form below or send us an email to cornerstone@biaseparations.com






