Sartorius BIA Separations
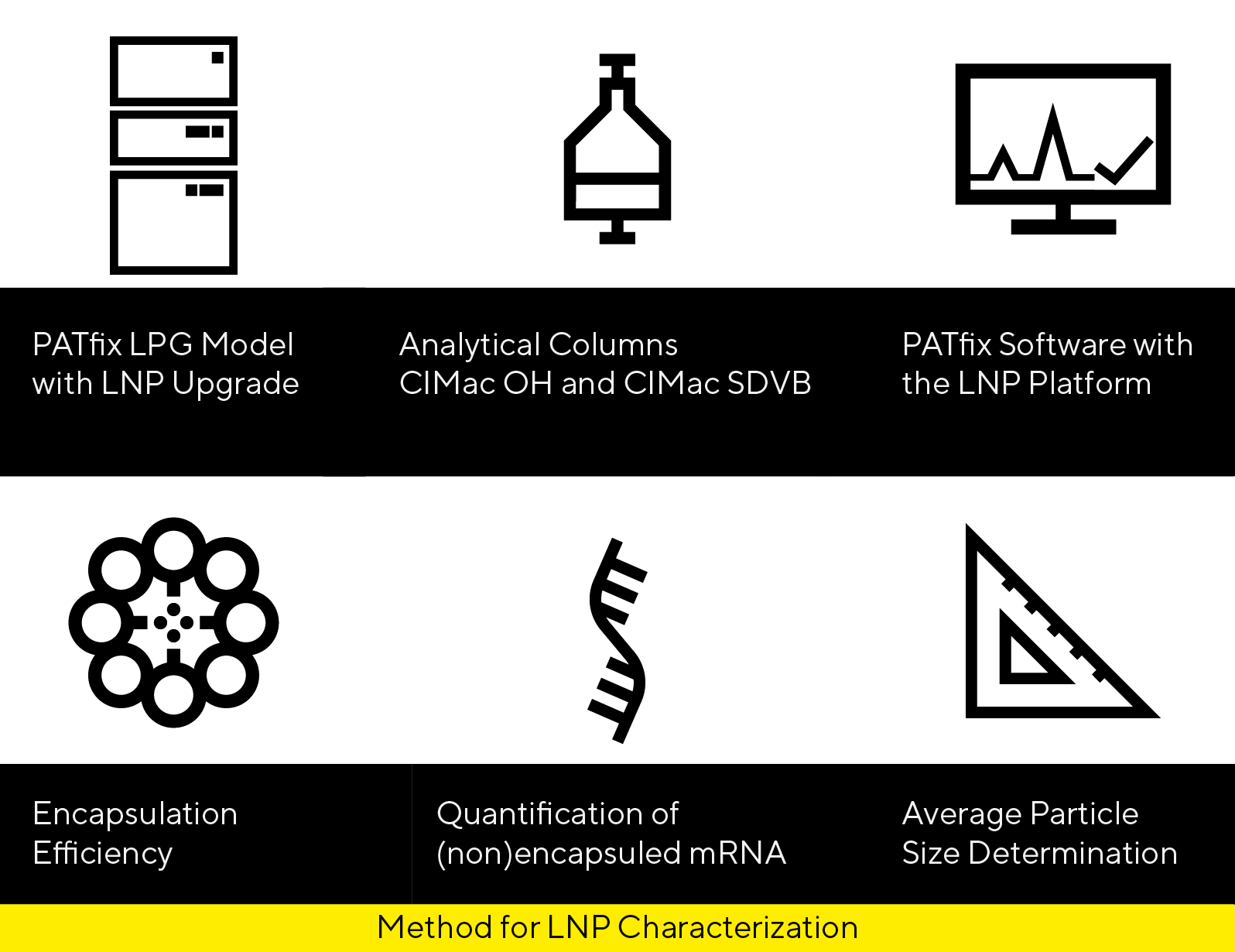
The PATfix® LNP Switcher Platform is an all-in-one analytical system built for lipid nanoparticle (LNP) analysis to accelerate the development of nucleic acid-based therapeutics. This chromatography-based analytics enable process development (PD) scientists to monitor their workflow in real time. PATfix LNP Switcher Platform includes carefully selected hardware components and analytical columns, combined with a software framework that integrates ready-to-use methods.
Characterize LNPs in a single run:
- measure encapsulation efficiency,
- quantify encapsulated and free mRNA,
- determine mRNA drug product integrity,
- evaluate particle size.
Discover Leasing Options for PATfix: Leasing allows you to acquire the PATfix without requiring CAPEX. Please contact sales@biaseparations.com for a quote or more information about leasing options.
Overview | PATfix® LNP Switcher Platform
Lipid nanoparticles (LNPs) have become the leading delivery vehicles for nucleic acid–based therapeutics, driving advances in mRNA vaccines and gene therapies. Their complexity, however, demands precise and reliable analytical tools to ensure quality, stability, and efficacy.
A chromatography-based analytical platform designed for lipid nanoparticle (LNP) characterization, enabling real-time separation, quantification, and process control to accelerate the development of nucleic acid–based therapeutics. It provides multiparameter insights into critical quality attributes such as encapsulation efficiency, nucleic acid integrity, nucleic acid–lipid adducts, particle heterogeneity, lipid composition, and purity. Supporting multiple nucleic payloads, the dual-column, valve-switching configuration enables the separation of free and encapsulated components while allowing real-time characterization of complex LNPs (eg. tLNPs), for CRISPR and CAR-T applications, and combination vaccines application. This innovation offers an orthogonal alternative to conventional assays, thereby enhancing process monitoring and quality control across a broad range of nucleic acid and protein-based therapeutic applications.
Read the article about simultaneous determination of encapsulation efficiency, nucleic acid integrity, and size of LNP formulations
![]() Article | Analysis of LNPs UsingPATfix LNP Switcher Platform
Article | Analysis of LNPs UsingPATfix LNP Switcher Platform
Handles Multiple Payloads – Simultaneously Compatible with a broad range of nucleic acid payloads, including mRNA, siRNA, and DNA, supporting versatile therapeutic development.
Direct Encapsulation Quantification – Enables rapid and precise measurement of encapsulation efficiency, critical quality parameters for LNPs
One-Step Payload Release – Simplifies the release of encapsulated nucleic acids, ensuring efficient and reproducible analysis.
Orthogonal to Existing Methods – Complements established techniques by providing independent measurements.
Advanced Insight into Formulations – Offers real-time separation and quantification, providing a deeper understanding of LNP integrity, stability, and performance.
Application of the PATfix LNP Switcher Platform
The LNP Switcher method accurately quantifies encapsulation efficiency in various LNP samples. Using a two-dimensional (2D) chromatographic setup with two complementary column chemistries, it effectively separates non-encapsulated nucleic acids from the encapsulated ones.
Challenge addressed:
Direct measurement of encapsulation efficiency from LNPs—no sample pre-treatment and no dyes required.
PATfix Solution:
2D-chromatography with UV detection enables precise encapsulation efficiency determination for single- or multipayload LNPs.
Detection
Multi-Wavelength UV Detector
Monitors multiple wavelengths of UV light to distinguish between free and encapsulated nucleic acids.
Up to 4 wavelengths (190–700 nm).
Conductivity I pH
Help to monitor salt concentration in real time and thus ensuring quality of the buffers.
0.1–999 mS/cm
pH measured range pH 2-12
Multi-Angle Light Scattering (MALS) Provides LNP particle size and heterogeneity measurements
Light Scattering Angles 28° – 156° at 9 angles
Molar Mass Range 103 to 109 Da depending on sample
Laser Specifications 660 nm (red)
CIMac OH
Ustilizinga hydrophilic, nonionic monolithic stationary phase, CIMac OH separates intact LNPs from free mRNA and excipients to enable accurate determination of encapsulation efficiency and assessment of sample.
- Separates LNP particles from non-encapsulated mRNA and formulation components
- Minimal method development: no ion-pairing reagents required
- Ideal for monitoring encapsulation and carryover of excipients across formulation screens
- Complements CIMac SDVB for comprehensive integrity profiling after LNP disassembly
CIMac SDVB
Using a styrene–divinylbenzene monolithic phase in reversed-phase mode (typically with RNA-appropriate ion-pairing conditions), CIMac SDVB profiles mRNA drug product integrity and quantifies free mRNA and shorter fragments following LNP disassembly.
- High resolution for intact mRNA versus fragments.
- Robust peak shape and reproducibility for quantitative workflows
- Complements CIMac OH by providing detailed mRNA integrity data after LNP disruption
The PATfix® software simplifies analytical chromatography for day-to-day operations while retaining the necessary detail and complexity for higher-level tasks:
- 21 CFR Part 11-compliant software with included pre-validated methods according to FDA | EMA guidelines
- Information extraction handled via user-defined templates
- Data visualization accelerates progress during process development
- A single database of chromatograms is created from multiple analytical systems
- Easily share interactive results with colleagues, customers, and regulators: report generation helps ease the paperwork load
Library
FAQ About PATfix LNP Switcher Platform
It separates and characterizes free and encapsulated nucleic acids within LNP formulations, providing encapsulation efficiency, nucleic acid integrity indicators, particle size and heterogeneity, lipid detection, and evidence of nucleic acid–lipid adducts.
No. The platform is designed for direct injection after simple dilution — no fluorescent dyes or chemical labels are required.
The system supports a broad range of nucleic payloads, including mRNA, siRNA, pDNA, gDNA, and mixed payload formats used in therapeutic LNPs.
PATfix offers direct, chromatography-based separation and orthogonal detection that consolidates the information typically obtained from several assays into one validated run — improving reproducibility, reducing sample handling, and providing richer interpretable data.
Yes. The included software is 21 CFR Part 11-compliant and supports secure data capture, audit trails, and standardized reporting for regulatory submissions.
In many use cases the PATfix LNP Switcher can replace data traditionally gathered by up to three separate techniques (DLS, fluorescence, CE) streamlining workflows across PD and QC.
You will get the analytic results in less than 45 minutes. Using autosampler you can load up to 2 x 384 samples on the system.
In the package you will get everything including CIMac monolithic columns (method-matched – CIMac SDVB and CIMac OH). Detail description of the buffer preparation should be followed in SOP that are in the platform.
You need minimum od 120 µL with 0.2 ng/µL of mRNA sample or around 500 ng of encapsulated nucleic acids.