Sartorius BIA Separations
The PATfix® mRNA Platform is designed to monitor mRNA production and purification processes, simplifying analytical chromatography for process development scientists. The platform setup enables reliable at-line insight into:
- IVT reaction monitoring
- mRNA quantification
- Final quality control for mRNA size, integrity, and detection of dsRNA
PATfix mRNA Platform includes everything you need for mRNA monitoring:
- Hardware: a biochromatography system with selected components
- Software: a dedicated PATfix mRNA framework
- Analytical columns: specialized for mRNA in-process monitoring
- Methods: fully optimized analytical methods for mRNA production
Application | Methods
Optimized analytical methods are a key component of PATfix. Even non-chromatography expert can run analytics in process lab using the three orthogonal methods, that include:
- Fully optimized and validated analytical methods (to run a sample and pre-set methods for analysis)
- Guidelines for buffer and sample preparation, including detailed SOPs
- An mRNA calibration standard, which enables accurate quantification of the mRNA species of interest as well as batch-to-batch and day-to-day performance tracking.
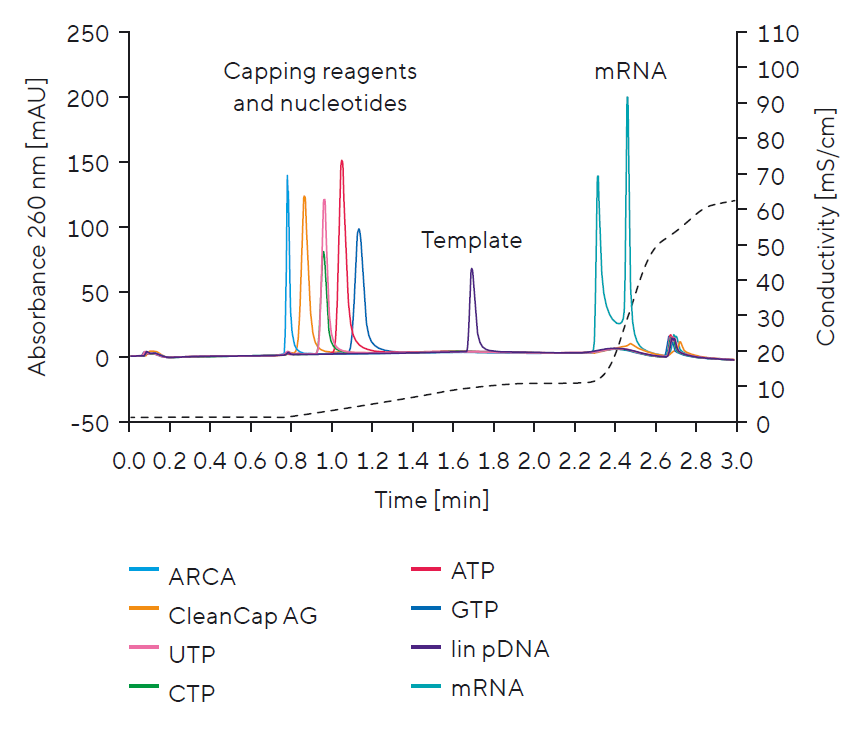
Optimizing the IVT reaction for high mRNA yield requires construct-specific considerations. To achieve this, all key components need to be separated and quantified. This enables rapid DoE execution to find optimal IVT conditions for a high yield mRNA production process. The PATfix® mRNA analytical platform is specifically designed to tackle these challenges.
Monitoring IVT Components:
- Individual nucleotide concentrations
- Including CTP and UTP
- Capping reagent
- DNA template
- mRNA product
Separation of IVT Components
Optimizing IVT Conditions:
- Buffer type and pH
- Salt(s) type and concentration
- Additives such as DTT
- T7 polymerase concentration
- Linear template concentration
- Reaction temperature
- Reaction time
Improving mRNA Yield With a Fed-Batch Approach to IVT
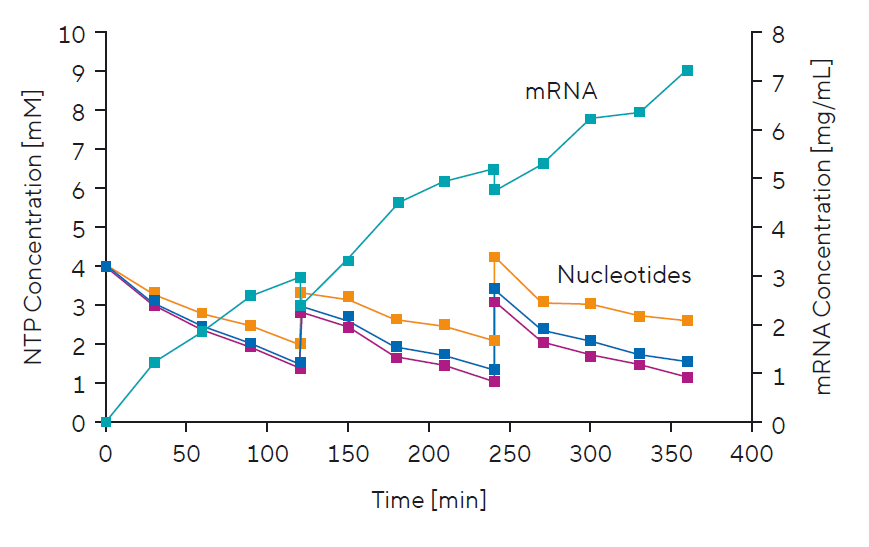
At-line mRNA production monitoring requires a unique blend of simplicity and information on the key process parameter — mRNA titre. The PATfix® mRNA platform can be implemented to monitor both processes, mRNA production and purification. This makes it a perfect analytical tool for in-process control. Speed is an important parameter when choosing process analytics; this method takes only 5 minutes, so process insights are almost instantaneous.
Simple and Fast mRNA Production Monitoring:
- mRNAs with polyA tail
- Process impurities (nucleotides, capping reagent, DNA template, abortive fragments, and enzymes)
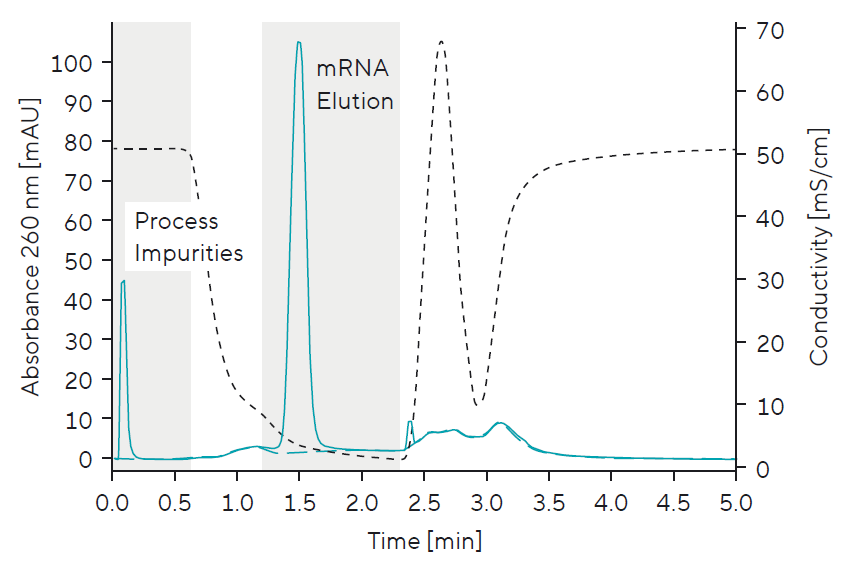
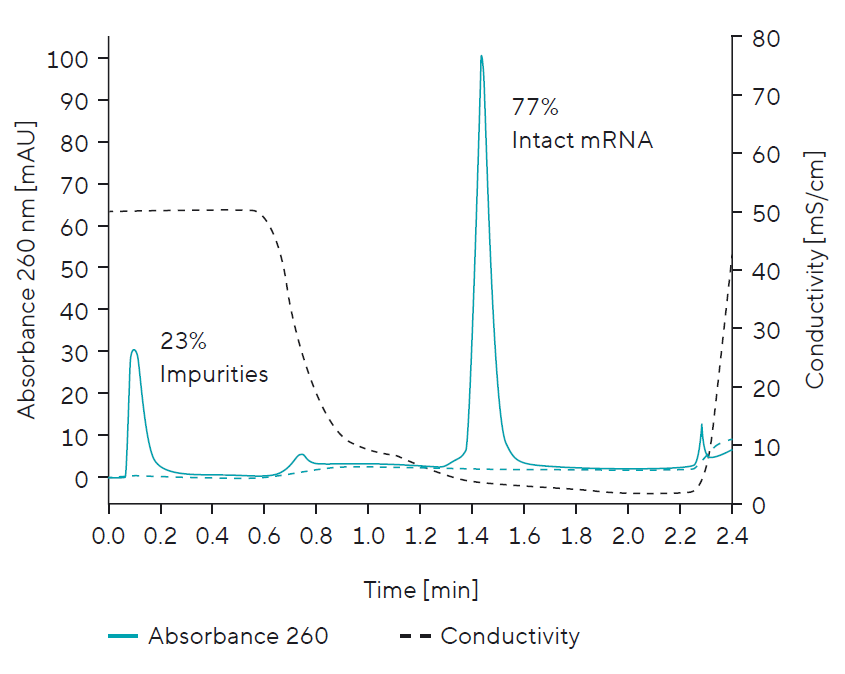
Quantification | Percentage of Intact mRNA vs. Impurities:
- Simple, fast at-line quantification
- Process step recovery monitoring (load, wash, elution, CIP fraction mass balance)
- Process yield monitoring
- Monitoring mRNA recovery vs. process impurity removal (nucleotides, enzymes, template, abort products)
Abortive transcripts, degraded | split mRNA, and dsRNA (e.g., due to 3′ extension) represent some of the main product related impurities that must be removed during the downstream process. Monitoring the same impurities during drug substance formulation and stability studies is equally important. A robust platform that considers these impurities — even during process development — can be of great value when quickly establishing quality control analytics.
Product-related impurity detection is crucial for:
- Developing process workflows (removal of impurities such as dsRNA)
- Process scale-up and validation
- Drug substance stability
Product impurity detection:
- Distinguish between drug substance and product impurities
- mRNA drug substance
- Product-related impurities
- dsRNA (e.g., due to 3′ extension)
- Abortive fragments
Size Separation to Distinguish Between Drug Substance (mRNA, 4,000 nt) and Process-Related Impurities
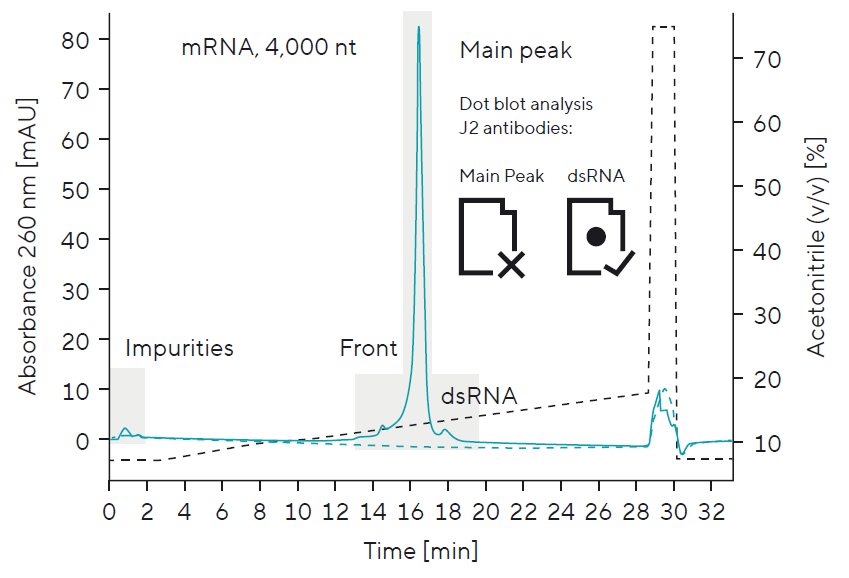
Separation by size allows for integrity studies:
- During processing | purification
- Final formulation
- Stability
- Storage
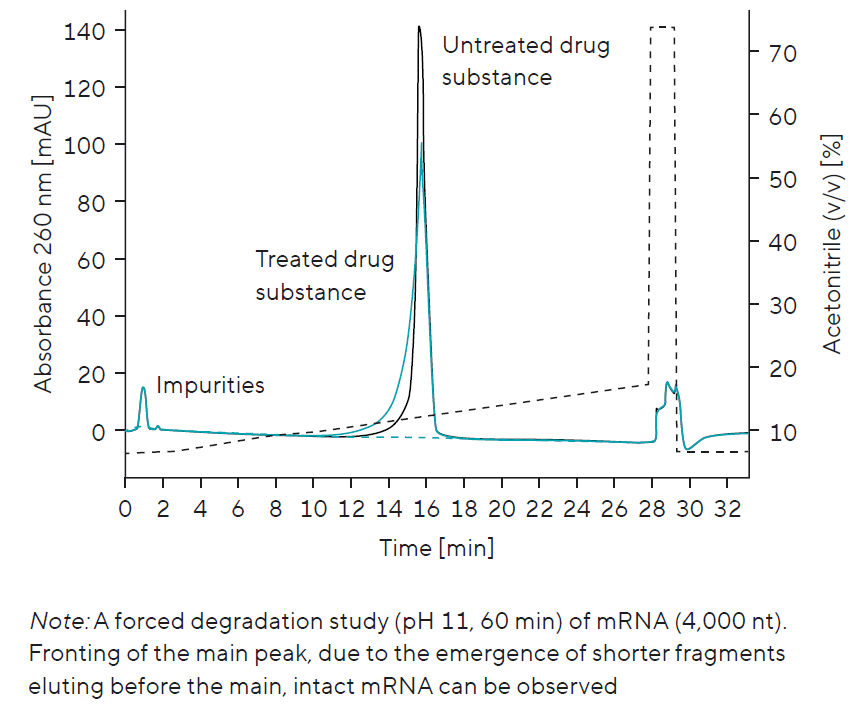
Stability Monitoring Using a Forced Degradation Study
Product Overview
PATfix® analytical system is all it takes to accelerate process development and have continuous at-line production monitoring and robust quality control of nucleic acid drug substances. The PATfix® analytical platform is designed to simplify analytical chromatography for non-experts, making it accessible for at-line use during process development.
The platform offers:
- The pre-set analytical system with the recommended detectors
- Methods together with SOPs to run samples and perform at-line analysis
- The appropriate CIMac analytical columns for mRNA process development and follow-up from IVT to purification
- mRNA standards for day-to-day system suitability testing (SST)
By bringing this tool from QC to at-line, feedback on process state is instantaneous (5–15 minutes per sample), accelerating process development workflows.
![]() Read a Customer Experience | WACKER Biotech
Read a Customer Experience | WACKER Biotech
The user-friendly PATfix® software simplifies data processing, visualization, and sharing.
All methods have been developed using CIMac analytical columns, known for
- multi-use design
- large flow-through channels
- high flow rates
- low-shear laminar flow
- convective mass transport
- flow-independent capacity and resolution
PATfix mRNA Platform includes three different CIMac chemistries.
CIMac Column for the Quantification of mRNA, Capping Reagents, and Nucleotides
A multimodal chromatography column using monolith technology quantifies the depletion of individual nucleotides, capping reagent, and generation of mRNA throughout an IVT reaction and subsequent drug substance purification.
CIMac Column for Titre Determination During mRNA Production
mRNA with a polyA tail binds to the affinity column under high salt conditions, while impurities (nucleotides, capping reagents, DNA template, enzymes) do not bind to the column and flow through. This allows rapid and simple at-line determination of mRNA titre, in less than 5 minutes.
CIMac for mRNA Integrity Characterization and Contaminants Detection During QC
A reverse-phase monolithic column used with an ion-pairing reagent. This analytical method uses an acetonitrile gradient at elevated temperatures to drive separation by size enabling detection of RNA fragmentation (stability) and dsRNA.
The PATfix® software simplifies analytical chromatography for day-to-day operations while retaining the necessary detail and complexity for higher-level tasks:
- 21 CFR Part 11-compliant software with included pre-validated methods according to FDA | EMA guidelines
- Information extraction handled via user-defined templates
- Data visualization accelerates progress during process development
- A single database of chromatograms is created from multiple analytical systems
- Easily share interactive results with colleagues, customers, and regulators: report generation helps ease the paperwork load
You can run your free trial in the cloud without any installation:
Designing a suitable hardware set-up for reproducible analytical separation of large biomolecules is not trivial. Complex IVT mixtures are composed of the target molecule (mRNA), process-related impurities (nucleotides, capping reagent, and DNA template), and product-related impurities (abortive fragments and dsRNA), all with similar biophysical characteristics. The PATfix® mRNA platform includes the preconfigured and carefully selected hardware components to realize the required analyses.