Cornerstone® Biomanufacturing Development Services
Are you seeking speed and scalability for your virus particle and large molecule process, from preclinical and PI to PIII and beyond?
Do you lack expertise and capabilities when it comes to AAV, pDNA or mRNA purification and analysis?
Are you looking to improve or fix an existing large biomolecule process?
No matter if you are a large company or a start-up. We have got your back!
Downstream part of the process is a complex matrix full of interdependent parameters where optimization is not straightforward, so experience provides the quickest way to identify the key quality attributes for your unique product and optimizing them for best performance. This allows for significant time, labour and opportunity cost savings, permitting your team to focus on bringing your product to clinic and market.
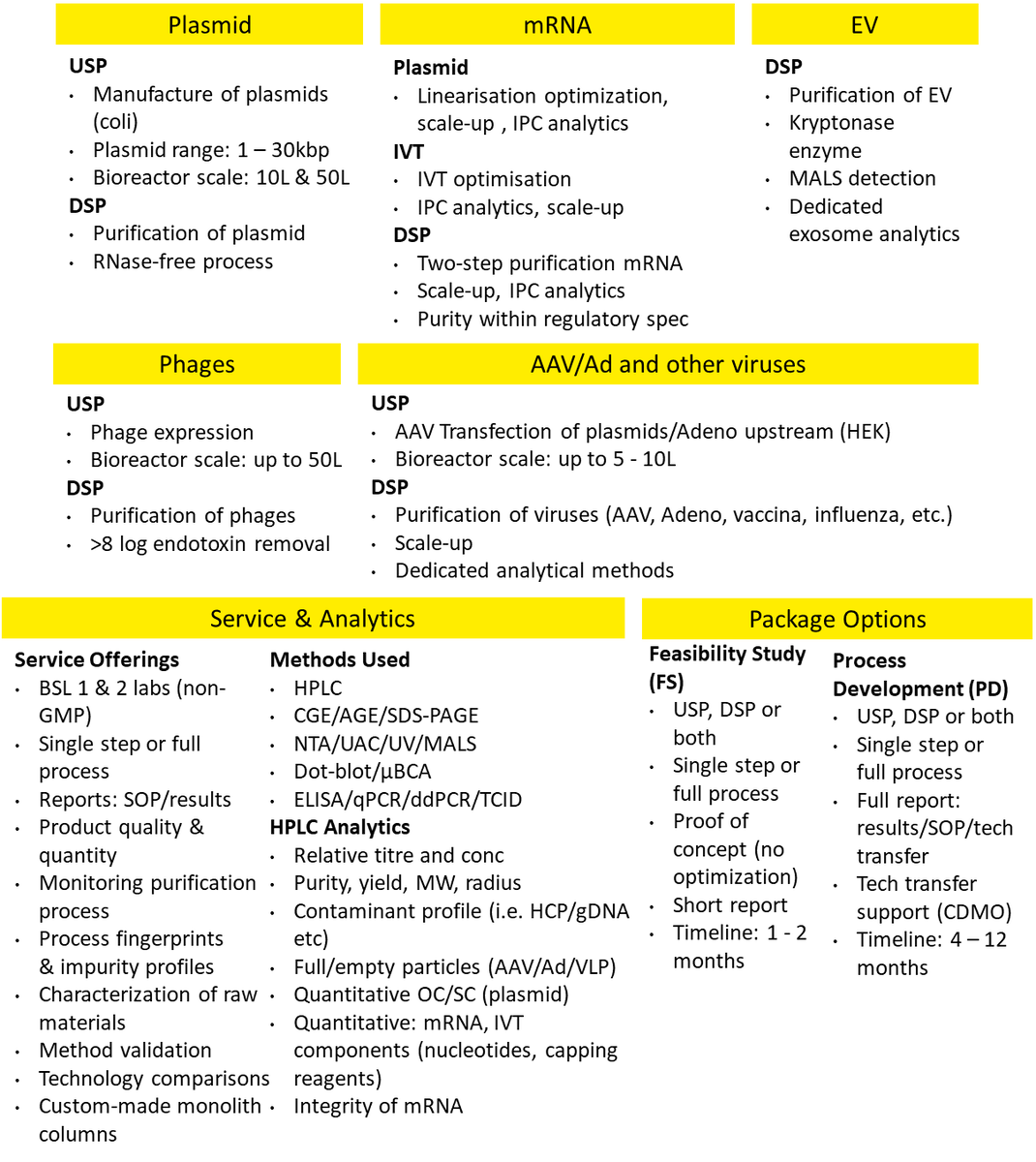
Our Cornerstone® Biomanufacturing Development Services are result of our more than 20 year of hands-on experience with the most challenging biopharmaceutical products and offer a comprehensive approach of integrated process development solutions and novel technology designed to improve the robustness and yield of AAV, pDNA, mRNA, exosomes and other Advanced therapy medicinal products (ATMPs) production, while improving the safety of therapeutic products.
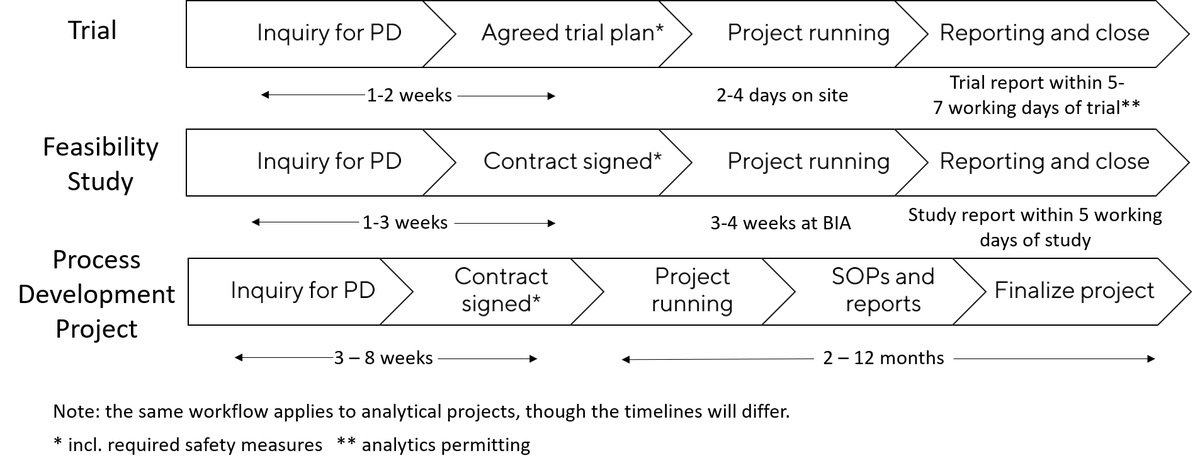
Reach out to us and have your Process Developed in 4 simple steps.
In our expanded, fully-equipped BSL 1 and 2 facilities, we can support you with process development, analytical method development, or preparation of custom purification media with your ligand of choice.

Better purification process. Faster.
Cornerstone® Biomanufacturing Development Services solve the challenges and accelerate process development from pre-clinical to market supply. Our objective is to give the customers resources, knowledge, technical solutions and their product - in time and in the right quality.
The Challenge and The Outcome.
Collaboration with AveXis led to the first licensed AAV therapeutic in the world. It took just 15 months from laboratory to manufacturing for market supply!
“We are especially grateful that Sartorius BIA Separations shared, and operated, with the same sense of urgency we did to help bring gene therapy to the SMA community. BIA’s experience with AAV purification and its chromatographic technology were important contributions and we look forward to our continued work together.”
Andy Stober, Former Senior Vice President of Technical Operations for AveXis
Our commitment to the advancement of mRNA therapies extends beyond supply of purification solutions. With open communication and close collaboration we support our customers in their path to develop and commercialise new therapeutics. Arcturus Therapeutics has been working closely with our scientists to support rapid development and deployment of their mRNA platform.
“We use Sartorius BIA Separations Monolith columns for the purification of our mRNA drug substance. The Monolith columns come in multiple sizes to meet our needs from small scale product development work to large scale cGMP manufacturing runs. We have found the Monolith columns to provide high throughput and high purity while being very robust and reliable. In addition, Sartorius BIA Separations has top notch customer service that never fails to impress.”
Greg Kubczak, Arcturus Therapeutics Director of Technical Services and Manufacturing.